Details of the Drug
General Information of Drug (ID: DMH75KV)
| Drug Name |
Acenocoumarol
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acenocoumarin; Acenocoumarolum; Acenocumarol; Acenocumarolo; Acenocumarolum; Acenokumarin; Ascumar; Minisintrom; Neositron;Nicoumalone; Nicumalon; Nitrovarfarian; Nitrowarfarin; Sincoumar; Sinkumar; Sinthrom; Sinthrome; Sintrom; Sintroma; Syncoumar; Syncumar; Synthrom; Syntrom; Zotil; Acenocoumarol Alliance Brand; Acenocoumarol Novartis Brand; Acenocoumarol [INN]; Acenocumarolo [DCIT]; Acenokumarin [Czech]; Alliance Brand of Acenocoumarol; Ciba Geigy Brand of Acenocoumarol; Mini Sintrom; Novartis Brand of Acenocoumarol; G 23350; Acenocoumarol (INN); Acenocoumarol Ciba-Geigy Brand; Acenocoumarolum [INN-Latin]; Ciba-Geigy Brand of Acenocoumarol; G-23350; Mini-sintrom; Sinthrome (TN); Sintrom (TN); AB-014/25000129; G-23,350; Mini-sintrom (TN); Nitrophenylacetylethyl-4-hydroxycoumarine; 2-hydroxy-3-[1-(4-nitrophenyl)-3-oxobutyl]-4h-chromen-4-one; 2-hydroxy-3-[1-(4-nitrophenyl)-3-oxobutyl]chromen-4-one; 3-(alpha-(4'-Nitrophenyl)-beta-acetylethyl)-4-hydroxycoumarin; 3-(alpha-(p-Nitrophenol)-beta-acetylethyl)-4-hydroxycoumarin; 3-(alpha-Acetonyl-4-nitrobenzyl)-4-hydroxycoumarin; 3-(alpha-Acetonyl-p-nitrobenzyl)-4-hydroxycoumarin; 3-(alpha-Acetonyl-para-nitrobenzyl)-4-hydroxy-coumarin; 3-(alpha-p-Nitrophenyl-beta-acetylethyl)-4-hydroxycoumarin; 4-Hydroxy-3-(1-(4-nitrophenyl)-3-oxobutyl)-2H-1-benzopyran-2-one;4-Hydroxy-3-[1-(4-nitrophenyl)-3-oxobutyl]-2H-chromen-2-one
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anticoagulants
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
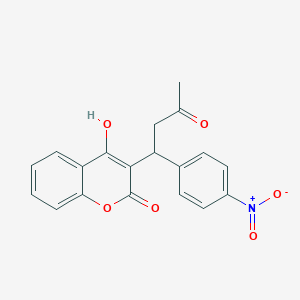 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 353.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
